The microbiome associated to the marine fanerogames, studies and applications
Seagrasses are benthic ecosystems that provide important services in the coastal zones, yet are declining worldwide at an alarming rate. Seagrasses provide habitat to commercially important fish and crustaceans, and contribute to coastal protection through the stabilization of sediment run-off. They play a critical role in the maintenance of biogeochemical cycles, burying approximately 12% of the global atmospheric carbon production. Rising anthrophogenic and natural threats, including human-induced climate change may negatively affect seagrasses, resulting in permanent habitat loss and reduction of biodiversity. With most of seagrass environmental monitoring based on long term responses to environmental pressures, there is a growing interest to develop alternative diagnostic tools that more effectively identify changes in seagrasses conservation status at an early stage. Diversified microbial communities are known to be associated with seagrasses above (leaves) and belowground (rhizomes and roots). These microbial communities are tightly associated with plants and have the capacity to adapt rapidly to changes in environmental conditions, helping secure meadow health and conservation. This research activity has been devoted to study the microbial communities of seagrass, to describe their structure and composition even in different environmental conditions. These studies give the necessary basic knowledge to understand the relationship microbiome/seagrass plants and their possible use as a putative marker of environmental change.
Next Generation Sequencing techniques has been applied (in the beginning, 454-pyrosequencing, then Illumina Platform) to the microbial metagenome associated with plants of Halophila stipulacea from Red Sea, Thalassia hemprichi from Faafu atoll (Maldives) and Posidonia oceanica from Cyprus and Crete. These analyses showed how different the microbial communities are, in both different plant parts and environmental conditions, varying along environmental gradients and according to the eco-physiological state of the plant. This depends on the ability of microbial communities to rapidly respond to environmental changes, and implies that the host plant/associated microbiome interaction plays a crucial role in the plant's fitness, but also that the host and all associated microorganisms represent a complex functional unit, called holobiont, which significantly changes the concept of living organism. Therefore, microbial communities are able to provide indications on the conservation status of plants and, consequently, could represent a sensitive putative monitoring tool and ecological indicator, to detect signs of stress in marine plants, before an irreversible decline occurs.
This work has been possible thanks to many collaborations, some of which are still active, such as the one with Gidon Winters of the Dead Sea & Arava Science Center, Hazeva (Israel); Pedro Beca Carretero of the Ryan Institute for Environment, Marine and Energy, NUI Galway (Ireland); Marlen Vasquez of the Cyprus Technological University, Limassol (Cyprus) and Eugenia Apostolaki of the Institute of Oceanography, Hellenic Center for Marine Research, Heraklion (Crete, Greece). The field studies were possible thanks to the numerous grants won on the ASSEMBLE, ASSEMBLE plus, COST and Erasmus projects financed by the European Community, and a MaRHE prize, awarded to Astrid Mejia, in 2014.
The most recent development of this project has been the methodological set up and the analysis of associated fungal community, which is also a promising source of information on the conservation status of seagrasses.
Frasca S., Alabiso A., D’Andrea M.M., Migliore L. (2024). UNCOVERING THE FUNGAL COMMUNITY COMPOSITION OF ALIVE AND DEAD POSIDONIA OCEANICA MATTE. Microbial Ecology, 87, 170. DOI: https://doi.org/10.1007/s00248-025-02492-6
Posidonia oceanica retains a large amount of carbon within its belowground recalcitrant structure, the ‘matte,’ which is characterized by low oxygen availability and biodegradation. Fungi may play a pivotal role in carbon sequestration within the matte, even if little/no information is available. To fill this gap, we profiled fungal communities from the upper and lower layers of alive and dead matte, by using an ITS2-5.8S rDNA metabarcoding approach. The study was conducted in a shallow coastal stretch of the Aegean Sea (Crete). Then, 184 operational taxonomic units were identified, predominantly belonging to Ascomycota, in alive and dead matte. Nevertheless, their composition significantly differed: the host-specific Posidoniomyces atricolor was dominant in alive but not in dead matte, while fast-growing saprotrophs, potentially accelerating the decomposition rate, increased in dead matte. These findings lay the groundwork for future investigations on the possible increase of biodegradation under the changing environmental conditions.
Frasca S., Alabiso A., D’Andrea M.M., Cattaneo R., Migliore L. (2024). DIVERSITY AND COMPOSITION OF POSIDONIA OCEANICA-ASSOCIATED BACTERIAL AND FUNGAL COMMUNITIES: EFFECT OF BOAT-INDUCED MECHANICAL STRESS IN THE VILLEFRANCHE-SUR-MER BAY (FRANCE). Diversity, 16(10), 604. DOI: https://doi.org/10.3390/d16100604
The anchoring and mooring of boats mechanically damage Posidonia oceanica plants; however, no information is available on the effect of this kind of damage on the plant holobiont, i.e., on the associated bacterial and fungal communities. Indeed, bacterial communities are known to change under different plant stress conditions but the dynamics of seagrass-associated fungi remain largely unexplored. We used DNA metabarcoding to profile the bacterial and fungal colonizers of two nearby P. oceanica patches in the Villefranche-sur-Mer bay (France) differing by the amount of exposure to mechanical stress due to boat transit and anchoring. Bacterial communities showed a significant reduction in diversity with an increase in Vibrio sp. in the rhizome and root samples from the impacted site, where the accumulation of dead organic material favors opportunistic heterotrophs. Conversely, fungal communities showed increased diversity in the leaf samples from the impacted site, where a reduction in the dominant P. oceanica host-specific mutualistic endosymbiont, Posidoniomyces atricolor, was found. This change was probably due to the opening up of new colonizable niches for several fungal species. Although this study represents a preliminary assessment of the effect of mechanical stresses on P. oceanica-associated microbial communities, it further supports their putative use as a seagrass descriptor.
Conte C., Apostolaki E.T., Vizzini S., Migliore L. (2023). A TIGHT INTERACTION BETWEEN THE NATIVE SEAGRASS CYMODOCEA NODOSA AND THE EXOTIC HALOPHILA STIPULACEA IN THE AEGEAN SEA HIGHLIGHTS SEAGRASS HOLOBIONT VARIATIONS. Plants, 12(2), 350. DOI: https://doi.org/10.3390/plants 12020350
Seagrasses harbour bacterial communities with which they constitute a functional unit called holobiont that responds as a whole to environmental changes. Epiphytic bacterial communities rapidly respond to both biotic and abiotic factors, potentially contributing to the host fitness. The Lessepsian migrant Halophila stipulacea has a high phenotypical plasticity and harbours a highly diverse epiphytic bacterial community, which could support its invasiveness in the Mediterranean Sea. The current study aimed to evaluate the Halophila/Cymodocea competition in the Aegean Sea by analysing each of the two seagrasses in a zone where these intermingled, as well as in their monospecific zones, at two depths. Differences in holobionts were evaluated using seagrass descriptors (morphometric, biochemical, elemental, and isotopic composition) to assess host changes, and 16S rRNA gene to identify bacterial community structure and composition. An Indicator Species Index was used to identify bacteria significantly associated with each host. In mixed meadows, native C. nodosa was shown to be affected by the presence of exotic H. stipulacea, in terms of both plant descriptors and bacterial communities, while H. stipulacea remained almost unchanged. This study provided evidence of the competitive advantage of H. stipulacea on C. nodosa in the Aegean Sea and suggests the possible use of associated bacterial communities as a descriptor of native seagrass sustainability.
Rotini A., Conte C., Winters G., Vasquez M.I., and Migliore L. (2023). UNDISTURBED POSIDONIA OCEANICA MEADOWS MAINTAIN THE EPIPHYTIC BACTERIAL COMMUNITY IN DIFFERENT ENVIRONMENTS. Environmental Science and Pollution Research, 30(42), 95464-95474. DOI: 10.1007/s11356-023-28968-x
Seagrasses harbour different and rich epiphytic bacterial communities. These microbes may establish intimate and symbiotic relationships with the seagrass plants and change according to host species, environmental conditions, and/or ecophysiological status of their seagrass host. Although Posidonia oceanica is one of the most studied seagrasses in the world, and bacteria associated with seagrasses have been studied for over a decade, P. oceanica’s microbiome remains hitherto little explored. Here, we applied 16S rRNA amplicon sequencing to explore the microbiome associated with the leaves of P. oceanica growing in two geomorphologically different meadows (e.g. depth, substrate, and turbidity) within the Limassol Bay (Cyprus). The morphometric (leaf area, meadow density) and biochemical (pigments, total phenols) descriptors highlighted the healthy conditions of both meadows. The leaf-associated bacterial communities showed similar structure and composition in the two sites; core microbiota members were dominated by bacteria belonging to the Thalassospiraceae, Microtrichaceae, Enterobacteriaceae, Saprospiraceae, and Hyphomonadaceae families. This analogy, even under different geomorphological conditions, suggest that in the absence of disturbances, P. oceanica maintains characteristic-associated bacterial communities. This study provides a baseline for the knowledge of the P. oceanica microbiome and further supports its use as a putative seagrass descriptor.
Winters G., Conte C., Beca-Carretero P., Nguyen H.M., Migliore L., Mulas M., Rilov G., Guy-Haim T., González M.J., Medina I., Golomb D., Kitson-Walters K. (2023). SUPERIOR BIOLOGICAL TRAITS OF INVADED (CARIBBEAN) VERSUS NATIVE (RED SEA) POPULATIONS OF THE SEAGRASS HALOPHILA STIPULACEA. Biological invasions, 25(7): 2325-2342. DOI: 10.1007/s10530-023-03045-z
The seagrass Halophila stipulacea is native to the Red Sea. It invaded the Mediterranean over the past century and most of the Caribbean over the last two decades. Understanding the main drivers behind the successful invasiveness of H. stipulacea has become crucial. We performed a comprehensive study including field measurements, a mesocosm experiment, and a literature review to identify ‘superior growth traits’ that can potentially explain the success story of H. stipulacea. We assessed meadow characteristics and plant traits of three invasive H. stipulacea populations growing off the Island of Sint Eustatius (eastern Caribbean). We compared similar parameters between native (Eilat, northern Red Sea) and invasive (Caribbean) H. stipulacea plants in a common-garden mesocosm. Lastly, we compared our field measurements with published data. The newly arrived H. stipulacea plants from St. Eustatius were characterized by higher percent cover, higher below and above-ground biomasses, more apical shoots, and faster leaf turnover rates than those measured in both native and older invaded habitats. These results were further confirmed by the mesocosm experiment where the invasive H. stipulacea plants grew faster and developed more apical shoots than the native plants. Results suggest that increased growth vigour is one of the main invasive traits that characterize successful invasive H. stipulacea populations in the Caribbean and potentially in other invaded areas. We encourage long-term monitoring of H. stipulacea in both native and invaded habitats to better understand the future spread of this species and its impacts on communities and their ecosystem functions and services.
Conte C., Rotini A., Winters G., Vasquez M.I, Piazza G., Kletou D., Migliore L. (2021). ELECTIVE AFFINITIES OR RANDOM CHOICE WITHIN THE SEAGRASS HOLOBIONT? THE CASE OF THE NATIVE POSIDONIA OCEANICA(L.) DELILE AND THE INVASIVE HALOPHILA STIPULACEA (FORSSK.) ASCH. FROM THE SAME SITE (LIMASSOL, CYPRUS). Aquatic Botany, 174: 103420. DOI: 10.1016/j.aquabot.2021.103420
Seagrasses and associated microbial communities constitute a functional unit (holobiont) which responds as a whole to environmental changes. However, it is still unclear how the microbial colonizers are selected. In this study we compared the epiphytic microbial communities associated with Posidonia oceanica and Halophila stipulacea, Mediterranean native and exotic seagrass species, respectively, growing side by side in monospecific patches within the port of Limassol (Cyprus, Eastern Mediterranean Sea). To evaluate whether the environment rather than the host species and/or its physiological condition play a role in shaping the seagrass epiphytic microbial community, the environmental microbial communities (seawater and sediment) and seagrass associated ones were determined by using 16S rRNA gene amplicon sequencing. Plant ecological status was evaluated by morphological (biometry), structural (density) and biochemical (pigment/phenol content) descriptors. In both species, leaf associated microbial communities are clearly similar to seawater microbes; conversely, microbes associated with H. stipulacea roots/rhizomes differ from the microbial communities in surrounding sediment. In both seagrasses, Pseudomonadaceae was the most abundant family on leaves, but each species harboured unique microbial families. To our best knowledge, this is the first study on these two neighbouring seagrass species, coupling plant ecological status with associated microbial communities. Results demonstrated that each seagrass responded differently to the same environmental conditions and selected different epiphytic microbial communities, supporting their putative use as ecological indicators.
Conte C., Rotini A., Manfra L., D’Andrea M.M., Winters G., Migliore L. (2021). THE SEAGRASS HOLOBIONT: WHAT WE KNOW AND WHAT WE STILL NEED TO DISCLOSE FOR ITS POSSIBLE USE AS AN ECOLOGICAL INDICATOR. Water, 13(4): 406. DOI: 10.3390/w13040406
Microbes and seagrass establish symbiotic relationships constituting a functional unit called the holobiont that reacts as a whole to environmental changes. Recent studies have shown that the seagrass microbial associated community varies according to host species, environmental conditions and the host’s health status, suggesting that the microbial communities respond rapidly to environmental disturbances and changes. These changes, dynamics of which are still far from being clear, could represent a sensitive monitoring tool and ecological indicator to detect early stages of seagrass stress. In this review, the state of art on seagrass holobiont is discussed in this perspective, with the aim of disentangling the influence of different factors in shaping it. As an example, we expand on the widely studied Halophila stipulacea’s associated microbial community, highlighting the changing and the constant components of the associated microbes, in different environmental conditions. These studies represent a pivotal contribution to understanding the holobiont’s dynamics and variability pattern, and to the potential development of ecological/ecotoxicological indices. The influences of the host’s physiological and environmental status in changing the seagrass holobiont, alongside the bioinformatic tools for data analysis, are key topics that need to be deepened, in order to use the seagrass-microbial interactions as a source of ecological information.
Rotini A., Conte C., Seveso D., Montano S., Galli P., Vai M., Migliore L., Mejia A. (2020). DAILY VARIATION OF THE ASSOCIATED MICROBIAL COMMUNITY AND THE HSP60 EXPRESSION IN THE MALDIVIAN SEAGRASS THALASSIA HEMPRICHII. Journal of Sea Research, 156: 101835. DOI: 10.1016/j.seares.2019.101835
The microbial communities associated with plant’s compartments and the expression of the chloroplast chaperonin Hsp60 have been simultaneously analyzed during the diel cycle in the marine seagrass Thalassia hemprichii from the lagoon of Magoodhoo island (Maldives), characterized by remarkable daily shifts in temperature and light. Plants showed a significant up-regulation of Hsp60 expression from 8.00 a.m to 2.00 p.m., in correspondence with the increase in temperature and light, confirming their role as defence mechanism against photoinhibition and oxidative damage. However, a further significant increase of the Hsp60 level was also observed when irradiance and temperature dropped, suggesting that the cellular stress was still in progress. The plant-associated microbial communities showed differences by plant compartment and sampling time, with the aboveground compartment (leaves) being much more dynamic than the belowground one (roots/rhizomes). In the phyllosphere, a progressive shift during the day from the absolute dominance of the Gammaproteobacteria class, mainly constituted of Enterobacteraceae family, to the increase of the biodiversity due to the rise of Alphaproteobacteria was observed. Belowground, the microbial diversity was much lower than aboveground, being Gammaproteobacteria the most represented class throughout all sampling times. Vibrionaceae family was the most abundant at 8:00 a.m. and 6.00 p.m. decreasing slightly at 2.00 p.m., partially replaced by Halomonadaceae. The combined biochemical and microbial markers allows to assess the plant stress response and to deepen the knowledge on the seagrasses adaptation to harsh and changing environmental conditions, resulting useful to detect early signs of change in an organism's physiological state. Furthermore, the variation of Hsp60 expression and associated bacterial communities in response to light and temperature fluctuations support the ‘holobiont’ theory, which considers the plant/microorganisms association as a functional unit, as suggested for corals.
Rotini A., Mejia A.Y., Costa R., Migliore L., Winters G. (2017) - THE SEAGRASS HOLOBIONT HALOPHILA STIPULACEA: ECOPHYSIOLOGICAL PLASTICITY AND BACTERIOME STRUCTURE ALONG A DEPTH GRADIENT IN THE NORTHERN RED SEA. Frontiers in Plant Science, 7: 2015
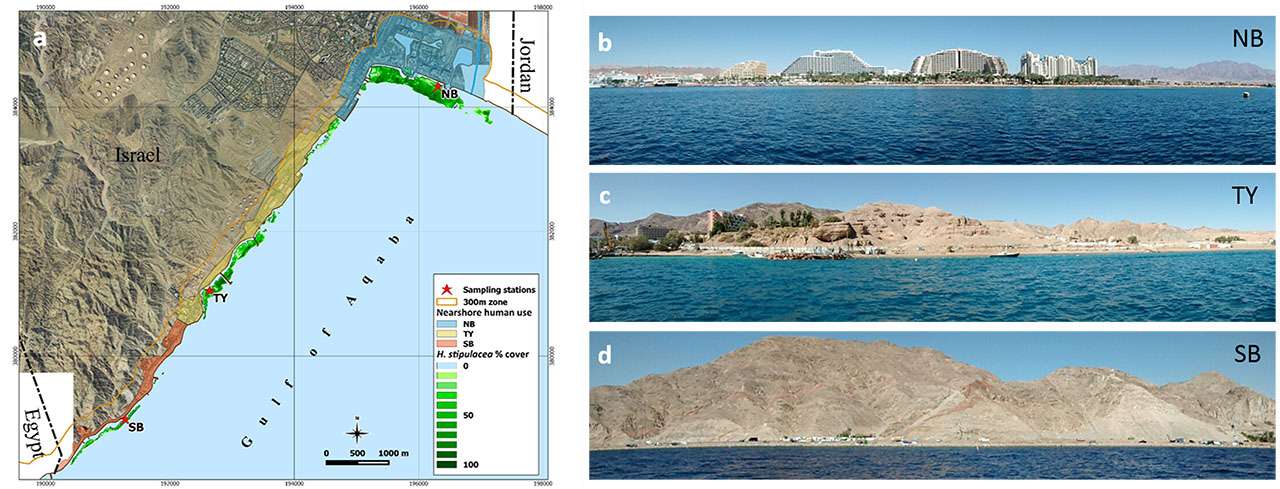
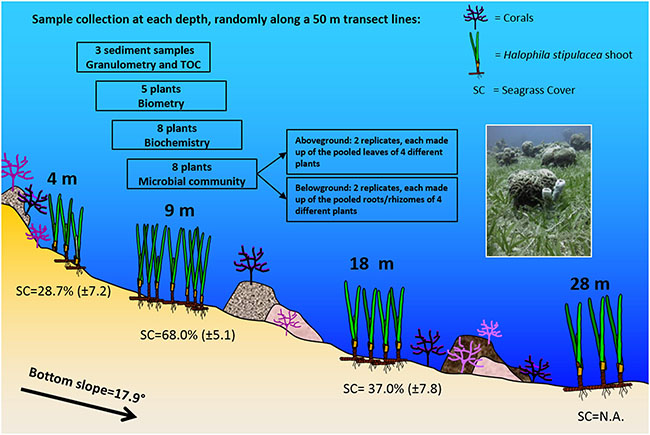
Halophila stipulacea is a small tropical seagrass species. It is the dominant seagrass species in the Gulf of Aqaba (GoA; northern Red Sea), where it grows in both shallow and deep environments (1–50 m depth). Native to the Red Sea, Persian Gulf, and Indian Ocean, this species has invaded the Mediterranean and has recently established itself in the Caribbean Sea. Due to its invasive nature, there is growing interest to understand this species’ capacity to adapt to new conditions, which might be attributed to its ability to thrive in a broad range of ecological niches. In this study, a multidisciplinary approach was used to depict variations in morphology, biochemistry (pigment and phenol content) and epiphytic bacterial communities along a depth gradient (4–28 m) in the GoA. Along this gradient, H. stipulacea increased leaf area and pigment contents (Chlorophyll a and b, total Carotenoids), while total phenol contents were mostly uniform. H. stipulacea displayed a well conserved core bacteriome, as assessed by 454-pyrosequencing of 16S rRNA gene reads amplified from metagenomic DNA. The core bacteriome aboveground (leaves) and belowground (roots and rhizomes), was composed of more than 100 Operational Taxonomic Units (OTUs) representing 63 and 52% of the total community in each plant compartment, respectively, with a high incidence of the classes Alphaproteobacteria, Gammaproteobacteria, and Deltaproteobacteria across all depths. Above and belowground communities were different and showed higher within-depth variability at the intermediate depths (9 and 18 m) than at the edges. Plant parts showed a clear influence in shaping the communities while depth showed a greater influence on the belowground communities. Overall, results highlighted a different ecological status of H. stipulacea at the edges of the gradient (4–28 m), where plants showed not only marked differences in morphology and biochemistry, but also the most distinct associated bacterial consortium. We demonstrated the pivotal role of morphology, biochemistry (pigment and phenol content), and epiphytic bacterial communities in helping plants to cope with environmental and ecological variations. The plant/holobiont capability to persist and adapt to environmental changes probably has an important role in its ecological resilience and invasiveness.
Mejia A., Rotini A., Lacasella F., Bookman R., Thaller M.C., Winters G., Migliore L. (2016) - MORPHOLOGY, BIOCHEMICAL DESCRIPTORS AND MICROBIAL COMMUNITY ANALYSIS: A PROMISING APPROACH FOR ASSESSING THE ECOLOGICAL STATUS OF SEAGRASSES. A CASE STUDY, HALOPHILA STIPULACEA FROM THE RED SEA. Ecological Indicators, 60: 1150-1163.
Seagrasses are one of the most valuable marine ecosystems on earth, yet they are declining worldwide at alarming rates. With most of seagrass monitoring based on long term responses to environmental pressures, there is growing interest in developing alternative diagnostic tools that more effectively identify changes in seagrass ecological status at an early stage. Besides morphological indicators, functional and biochemical descriptors may provide a good understanding of plant's responses to environmental changes. Moreover, the epiphytic microbial communities of seagrasses may also shift in response to changes in environmental conditions, although these have been seldom used as a descriptor of environmental change. In this study three Halophila stipulacea (Forsk.) Aschers meadows, found in the Gulf of Aqaba (northern Red Sea), were characterized using an integrated approach to highlight possible differences in the meadows ecological status. Plant descriptors, including leaves morphometrics (leaf size, leaf number/plant, leaves with lost apex), photosynthetic pigments (Chlorophylls, Carotenoids) and total phenols contents, were investigated and coupled with the plants’ epiphytic microbial community structure and composition, studied using pyrosequencing. The entire suite of descriptors highlighted differences among the meadows ecological status based on changes in plants’ morphology and biochemistry, and their associated microbial communities, in response to the different environmental conditions (water column turbidity, seawater and sediment nutrients) and the geomorphological features (bottom slope, granulometry) of the stations. Leaf morphology and photosynthetic pigment content were modulated in H. stipulacea in response to light availability and hydrodynamics in the Gulf of Aqaba. The highest leaf surface area and photosynthetic pigment contents were observed at the lowest irradiance and hydrodynamics/granulometry among stations. Total phenol content showed differences among stations with increasing concentrations from north to south. The microbial communities showed differences among stations and plant compartments, with high incidence of Gammaproteobacteria and Bacteroidetes in light limiting conditions, while Cyanobacteria and Rhodobacteraceae thrived in conditions of high light availability and hydrodynamics. The mutual response of the seagrass plants and the microbial communities provided evidence of their functional relationship, which undoubtedly needs further investigation. To the best of our knowledge, this is the first time that such descriptors have been used in an integrated approach. We provide evidence of their effectiveness in discriminating seagrass ecological status, even at small spatial scales. This work constitutes a new approach to the assessment of seagrasses and a stepping stone in the application of microbial communities as a putative marker in a changing environment.